A Period 2 Nonmetal With 6 Valence Electrons
What element has 6 valence electrons and 2 energy levels. Valence electrons refer to any electrons that an atom contains that are not in a full orbital.

Periodic Table And Valence Electron Quiz In 2022 Periodic Table Physical Science Electrons
Period 4 Group 15 element.

. The last of the noble gases 6. Period 4 group 5A element c. For the elements in the second period of the periodic table principal energy level n2 the s 2 p 6 electrons comprise the octet and no d sublevel exists.
Silicon 14 protons Group 4A 3rd period metalloid 4 valence electrons. 2 valence electrons 3 orbitals. Identify a nonmetal with 1 valence electron.
Identify the least reactive metal in period 3. The second element in Group 15 d. How many electrons are present in the 6 period.
Identify the nonmetal in period 2 that is more reactive than nitrogen and less reactive than fluorine. _____ _____ _____ _____ A transition metal with atomic mass of 1838used in incandescent light bulbs. All have 6 VALENCE ELECTRONS electrons in the outer energy level Oxygen sulfur and selenium are NONMETALS.
There are multiple elements that have six valence electrons including oxygen and sulfur. Period 2 nonmetal with 6 valence electrons. Most reactive metal in period 3.
Why is the Periodic Table Arranged the Way it Is. Valence Electrons Hydrogen 1 Metals Non-Metals and Valence Electrons Valence Electrons are the 01tennost electrons m an atom Each group column has the same number of valence electrons. Metaloid -3 Valence Electrons 5th Period.
Upgrade to remove ads. Up to 24 cash back Period. 2 8 6 1s2 2s2 2p6 3s2 3p4.
A period 2 nonmetal with 5 valence electrons. Aperiod 2 nonmetal with 5 valence electrons Other questions on the subject. The noble gas with 6 energy levels a.
_____ _____ _____ _____ a. The three well-known examples of the insulator are nitrogen sulphur and Neon. A metal with 4 protons.
These elements can be found in the sixteenth group in the vertical column of the periodic table also known as the chalcogens. Sulphur has 6 valence electrons 3. Only valence electrons are Involved m chemical bonding Lithium 6 27 Octet Rule Atom are more stable that haiœ a full shell of electrons.
The last of the noble gases. Economics Final Exam Questions and Answers. -4 or 4 common charge 4 valence electrons Period 5.
7 valence electrons 2 orbitals. What are the 6 metalloids on the periodic table. A transition metal use in incandescent light bulbs.
As a result the second period elements more specifically the nonmetals C N O F obey the octet rule without exceptions. Period 2 nonmetal with 6 valence electrons c. Transition metal number 24 A solid halogen with 5 energy levels Family 3A has this name.
The second element in Group 15. The non-metals are a bad conductor of electricity. What are the period 2 nonmetals with 6 valence electrons.
An element behaves as a non-metal when the number of valence electrons in its atom is more than four. A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. These elements are called insulators.
Oxygen is located in group 16 on the periodic table so it has six valence electrons. Period 2 nonmetal with 6 valence electrons c. Element with atomic number 31.
Sulfur 16 protons Group 6A 3rd period nonmetal 6 valence electrons E Config. Period 4 Group 15 element c. Oxygen is the only nonmetal with 6 Valence Electrons in period 2.
Phosphorus 15 protons Group 5A 3rd period 5 valence electrons E config. A transistion metal used in incandescent light bulbs. The element that has properties similar to Antimony and is in period 2.
Elements that have properties of both metals and nonmetals and touch the zigzag line except Al. The energy of the 6d subshell is even higher than that of the 7s subshellIn the 6th periodselectrons can be filled in only 6s4f5d and 6p subshells. The last of the alkaline earth metals.
What is the velocity of a 850kg car after starting at rest when 13000j of work is done to it. Period 3 element with 6 valence electrons. Tellurim and polonium are METALLOIDS.
Period 3 element with 6 valence electrons. A transition metal with 74 protons b. The periodic table is laid out in rows to illustrate recurring trends in the chemical behavior of the elements as their atomic number increases.
The last of the noble gases. Using the work energy theorem. 2 8 5 1s2 2s2 2p6 3s2 3p3.
The second element in group 5A Period 3 element with 6 valence electrons 22. Period 2 nonmetal with 6 valence electrons. What element in period 2 has 6 valence electrons.
Physics 22062019 1000 Trinhphuongtran. A new row is started when chemical behavior begins to repeat creating columns of elements with similar properties. The first element in the actinoid series.
One object has a mass of 1. Physics 22062019 0030 juansantos7b. All have 6 VALENCE ELECTRONS electrons in the outer energy level Oxygen sulfur and selenium are NONMETALS.
Common Charge 3 Period 5. Nitrogen has 5 valence electrons 2.
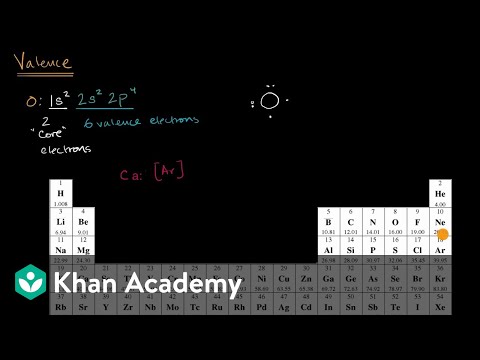
Valence Electrons Video Khan Academy

Color Coding The Periodic Table Groups And Periods Periodic Table Coding Electron Configuration
0 Response to "A Period 2 Nonmetal With 6 Valence Electrons"
Post a Comment